The intrinsic internal energy U is the total internal energy minus the energies of motion, gravitational, magnetic and surface forces energies . The first law can be written using U as
δQ = δU + δW
This is termed the restricted energy conservation equation for a system The notes below relate to various non flow processes using the restricted energy conservation equation when the kinetic and potential and surface energies are assumed zero.
The system boundaries are defined by the red dashed lines.
Heating at constant volume

δW= 0 therefore
δQ= δU
Adiabetic expansion in a cylinder

δQ= 0 therefore
δW= -δU
Free Expansion (Joules experiment)
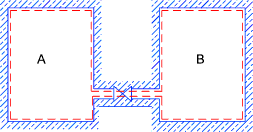
Initially valve is closed and pA > pB >
Valve is opened and pressures equalise.( Q = W = 0) E is constant throughout process
When conditions settle U = E therefore final U = initial U
Throughout path of proces U is not constant.
Heating at constant pressure

δQ= δU + δW
If the expansion is fully resisted (reversible process) δW= pδV therefore
δQ= δH
Note:
δH = δU + pδV + Vδp. In the above system p is constant (δp = 0). Therefore δH = δU + pδV